As we look to move away from fossil fuels, there is a strong interest in using hydrogen to carve out the future in certain ways. There has been growing hope that hydrogen can replace heavily polluting fuels in many sectors and power various industries and sectors. Since hydrogen is the most abundant element in the universe, this seems a logical idea, though as usual we shall see in this article how it is not so straightforward(1).
To produce energy, a ‘fuel cell’ uses the chemical energy of hydrogen to cleanly generate electricity. The fuel cell consists of a positive electrode (cathode) and negative electrode (anode) surrounded by a solution (electrolyte). When the hydrogen fuel is input at the anode, the molecules have their electrons stripped away leaving protons (hydrogen nuclei) behind. The protons flow through the electrolyte to the cathode, where they combine with oxygen atoms to produce water. The electrons however flow through a circuit, producing electricity(2). This process can be seen in the animation below.
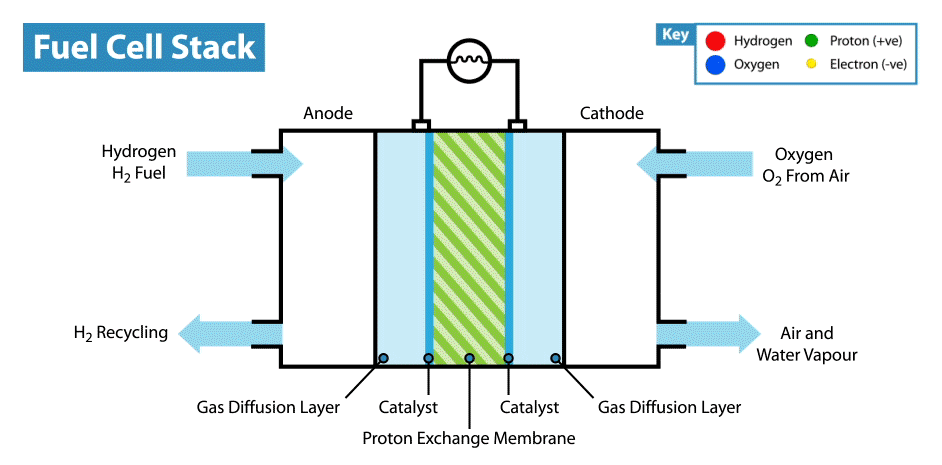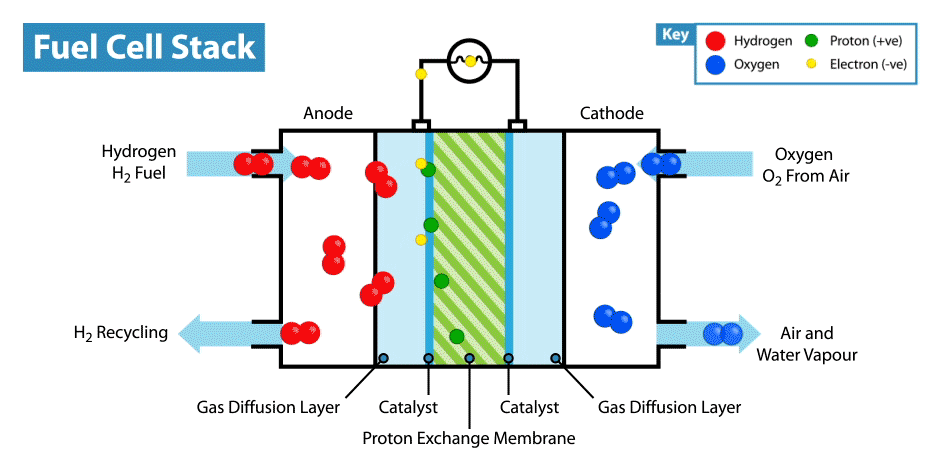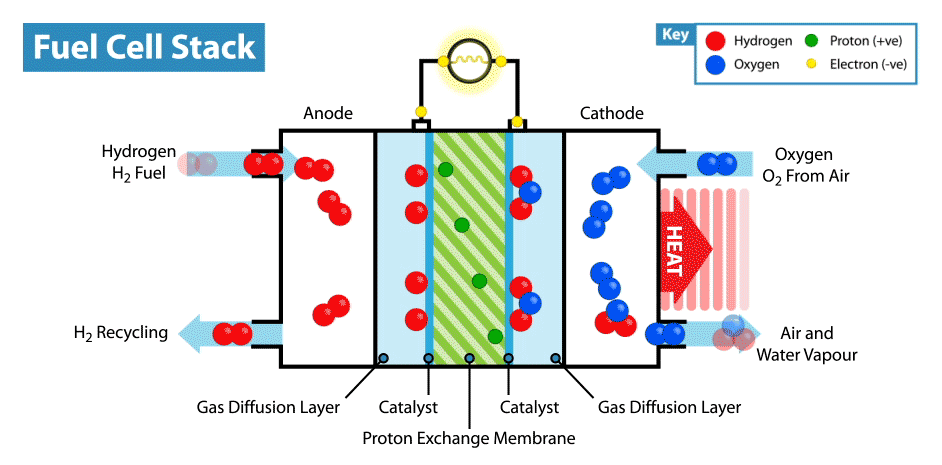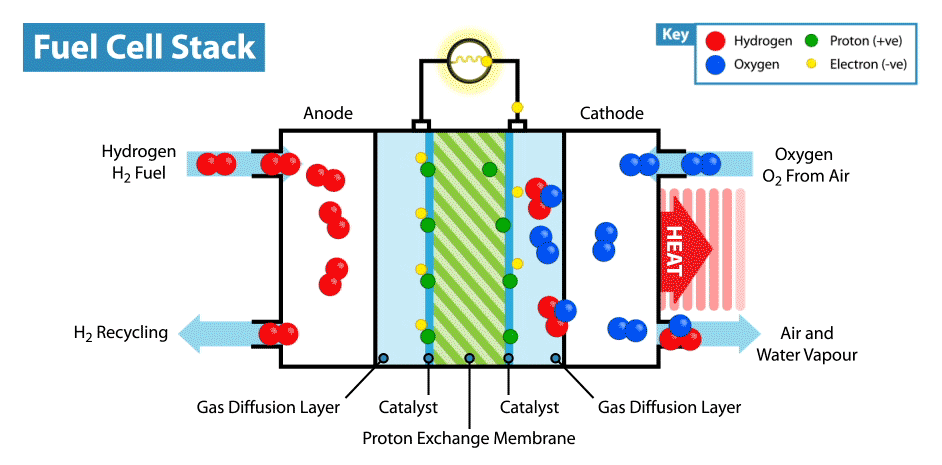
Hydrogen fuel cells therefore produce no carbon dioxide (or other greenhouse gases), and their only by-product is water. Meanwhile our other methods of producing energy such as burning fossil fuels (which accounts for about 80% of the global energy production) produces significant amounts of carbon dioxide, contributing heavily to global warming(1). This apparent cleanliness of hydrogen power is not the only reason why interest in hydrogen is gaining traction.
Hydrogen has many benefits and uses, and along with its potential emission-free use, many countries have adopted hydrogen in their plans to reach carbon neutrality. One of the main benefits of hydrogen is that it is able to help decarbonise certain sectors that are proving difficult to decarbonise through other means (such as ‘electrifying’ them)(4). For example, long-distance transport is proving hard to electrify due to the limited range and long recharging times associated with electric vehicles, whereas hydrogen fuel cell powered vehicles avoid these problems altogether. As a result, several countries are testing hydrogen powered buses(5). Aviation is another key sector for which hydrogen power seems very promising, with reports suggesting hydrogen-powered aircraft can reduce emissions significantly in the long term(6). It is thought that hydrogen power can help these tricky industries achieve net zero, while other means of power could not(4).
Another major benefit of hydrogen is that it is able to store excess renewable energy power which we could not otherwise do. On a particularly sunny or windy day, we imagine we would try and capture as much solar and wind energy as possible to store for the cloudy and still days when we cannot generate so much electricity. However a big issue with renewable energy currently is that it is incredibly difficult to store excess electricity that is not being used, and to try and do so can be very expensive(7). Hydrogen offers a way to solve this problem. If excess renewable electricity is used to produce hydrogen through electrolysis (we shall see how this works later), which can be stored and transported relatively easily, this can then be used when required to provide further clean power(1, 4). This hydrogen is flexible too and offers multiple uses, for example the hydrogen can be used in a fuel cell, be processed further to produce methane, be converted back into electricity or even supplement demand for natural gas in the heating/power sector. Among other benefits, these show why hydrogen is experiencing a surge of interest in the fight against global warming(1).
On the surface then, hydrogen seems the ideal solution for reducing emissions and thus global warming. Should we be converting everything we can to hydrogen power? Firstly however, we must consider the drawbacks of hydrogen. The main concern is that the hydrogen industry in its current state is not as clean as it appears. Hydrogen gas (H2) which is used in fuel cells does not exist naturally in significant amounts. Therefore we have to synthesis the pure gas using other methods, which is where the problems begin. Our current method of hydrogen production that produces 95% of the world’s hydrogen is called ‘steam reforming’, which is an energy intensive process using natural gas (methane – a fossil fuel)(8). The main reaction can be seen as follows:
![]()
However, a subsequent reaction occurs:
![]()
The final step is the main problem, as carbon dioxide is produced. This is not an insignificant amount either, for every 1 kg of hydrogen produced, 9.3 kg of carbon dioxide is produced(9). So while using hydrogen as a fuel is clean, producing the hydrogen is far from it. This doesn’t mean this is the end of the road for hydrogen power however, as there are much cleaner methods of producing hydrogen.
One way of making hydrogen production more environmentally friendly is to combine our existing method with carbon capture and storage (CCS) so the carbon dioxide produced can be stored long term or used for other means, keeping it out of the atmosphere. Hydrogen produced this way is called ‘blue hydrogen’. For this to be a viable option for producing hydrogen in the future however, we need to first assess how successful and easy to scale up CCS is, as the technology is not yet proven at large scales (as touched on in my previous article)(10).
Alternatively, we can produce ‘green hydrogen’ using electrolysis of water, an even cleaner method since no carbon dioxide is produced in the entire process. In this method, electricity generated from renewable sources (an important aspect) is used to split water up into hydrogen and oxygen, similarly to how a fuel cell works with an anode and cathode separated by an electrolyte. When water is input at the anode, the following reaction occurs(11):
![]() (Note: e– denotes an electron)
(Note: e– denotes an electron)
The electrons flow in an external circuit, and the hydrogen ions then move across to the cathode where they combine with the electrons to form hydrogen gas in the following reaction:
![]()
Overall, the following reaction occurs, showing that the process is entirely carbon dioxide free:

The process is depicted in the figure below(11).

Though this method seems on paper to be the best way of producing hydrogen, it is currently used only on a relatively small scale. The reason for this? It’s expensive. Electricity is more expensive than gas, and since the electrolysis process isn’t particularly energy efficient, hydrogen produced by electrolysis can currently cost around six to eight times the price of hydrogen produced via steam reforming using natural gas. Unfortunately this means that the vast majority still purchase and use the cheaper hydrogen, even though it increases the level of carbon dioxide in the atmosphere. Ultimately, to incentivise the use of the cleaner hydrogen, it needs to be more cost effective(10).
As the cost of hydrogen is heavily influenced by the price of the fuel used to produce it, one way to make green hydrogen cheaper is to reduce the cost of renewable energy(12). Fortunately as technology progresses, this is happening. In particular, solar and wind power costs have been decreasing rapidly in recent years. In a report by the International Energy Agency (IEA)(12), they argue how building electrolysers in locations with prime conditions for renewable energy generation can become a low-cost supply option for hydrogen for the future. This still holds even when factoring in logistical costs of storage and transport of hydrogen from the often-remote locations of renewable energy generation. The map below shows the long-term cost of hydrogen when produced from solar and wind systems, showing the regions such as Central Asia and parts of Africa and South America where hydrogen is expected to have particularly low costs. These areas may end up being optimal locations to produce hydrogen at a low cost for the future.

All in all, as the renewable energy to produce hydrogen becomes cheaper and fossil fuels become more scarce and thus expensive, green hydrogen will become cheaper and more widely used than traditionally produced hydrogen, benefiting the planet immensely.
Overall therefore it seems that hydrogen has great potential to reduce global warming in the future, and as a result, many countries are implementing hydrogen into their climate change policies. To make hydrogen successful however, they must proceed sensibly; hydrogen should not be expected to be the be-all and end-all when it comes to reducing global warming. For example, while hydrogen powered vehicles seem promising for long-distance, heavy transport and planes (among others), the technology currently seems less suited to passenger cars. Electric vehicle (EV) technology is much further advanced at this stage than hydrogen fuel cell vehicles, and EVs have surged in popularity in the past few years(4). Despite this, as hydrogen infrastructure develops, we may find ways to adapt technology to utilise hydrogen in ways we did not consider before.
An example of how this is happening as we speak, one group recently found viable methods of converting electricity to ‘negative-emissions hydrogen’. In other words, they have adapted the electrolysis process to use captured CO2 and convert it to other useful products along with the hydrogen. This is key as it has now been realised that to prevent global warming from reaching even more critical levels, ‘negative emissions’ are needed; taking carbon dioxide out of the atmosphere, which this method of hydrogen production claims to do(13).
We may even develop further ways to cleanly synthesise hydrogen that can supplement electrolysis. Other clean methods to produce hydrogen would be welcome as to produce the world’s hydrogen using electrolysis would require an extremely large amount of electricity(4). One example is ‘turquoise hydrogen’, produced by breaking down natural gas using high temperature to produce not only hydrogen, but highly sought-after materials, as well as producing no carbon dioxide(4, 14). As these technologies and methods progress, accessing hydrogen will become cheaper allowing us to further reduce our emissions and help our planet.
Author: Oliver Pearce
Note: There are a great deal of publications surrounding hydrogen, far too many to encompass in one article, to find out more please see the Further Reading page.
Bibliography:
- Simon Evans JG. Does the world need hydrogen to solve climate change?2020 03/09/2021. Available from: https://www.carbonbrief.org/in-depth-qa-does-the-world-need-hydrogen-to-solve-climate-change.
- Office of Energy Efficiency & Renewable Energy. Fuel Cells [Available from: https://www.energy.gov/eere/fuelcells/fuel-cells.
- Intelligent Energy. Hydrogen Fuel Cells 2021 [Available from: https://www.intelligent-energy.com/our-products/stationary-power/fuel-cells/.
- van Renssen S. The hydrogen solution? Nature Climate Change. 2020;10(9):799-801.
- Staffell I, Scamman D, Velazquez Abad A, Balcombe P, Dodds PE, Ekins P, et al. The role of hydrogen and fuel cells in the global energy system. Energy & Environmental Science. 2019;12(2):463-91.
- CSIRO. Opportunities for hydrogen in commercial aviation. 2020.
- World Nuclear Association. Electricity and Energy Storage 2021 [Available from: https://world-nuclear.org/information-library/current-and-future-generation/electricity-and-energy-storage.aspx.
- Rapier R. Life Cycle Emissions of Hydrogen 2020 [Available from: https://4thgeneration.energy/life-cycles-emissions-of-hydrogen/.
- Rapier R. Hydrogen Prodution with a Low Carbon Footprint 2020. Available from: https://www.forbes.com/sites/rrapier/2020/07/10/hydrogen-production-with-a-low-carbon-footprint/?sh=5ffc54f67c2c.
- Citu. Could hydrogen replace natural gas to heat homes in the UK? [Available from: https://citu.co.uk/citu-live/could-hydrogen-replace-natural-gas-to-heat-homes-in-the-uk.
- Office of Energy Efficiency & Renewable Energy. Hydrogen Production: Electrolysis [Available from: https://www.forbes.com/sites/rrapier/2020/07/10/hydrogen-production-with-a-low-carbon-footprint/?sh=5ffc54f67c2c.
- IEA. The Future of Hydrogen. Paris: IEA; 2019.
- Rau GH, Willauer HD, Ren ZJ. The global potential for converting renewable electricity to negative-CO2-emissions hydrogen. Nature Climate Change. 2018;8(7):621-5.
- Leal Pérez BJ, Medrano Jiménez JA, Bhardwaj R, Goetheer E, van Sint Annaland M, Gallucci F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. International Journal of Hydrogen Energy. 2021;46(7):4917-35.