CRISPR in the clinic
Current clinical trials utilizing CRISPR are still in their early development, meaning that even if the technology turns out to be safe and effective, CRISPR-based therapy is still far from being used by the wide public.
CRISPR technology represents an important advancement in personalized medicine. The current clinical targets are blood disorders, cancers, eye disease, chronic infections, and protein-folding disorders. All current clinical trials focus on editing somatic cells or tissues without affecting the sperm or eggs, meaning genomic changes will not be passed down to the next generations.
Two genetic blood disorders are caused by mutations affecting the haemoglobin gene: sickle cell disease (SCD) and beta-thalassemia. CRISPR technology does not directly change the mutation but rather increases the levels of fetal haemoglobin. Fetal haemoglobin can be used in the treatment of SCD and beta-thalassemia by substituting the defective haemoglobin of adults. Stem cells are harvested from the patient’s blood and CRISPR is used to edit the genomes of the harvested blood cells. Chemotherapy is used to eliminate the defective marrow stem cells and the newly genome-edited stem cells are put back into the patient’s bloodstream, resulting in blood cells producing fetal haemoglobin.
Encouraging results were obtained using ex vivo CRISPR-based therapy to treat beta-thalassemia in February 2019 and SCD in July 2019. The treated patients will be monitored for possible harmful effects. The main limiting factor until now has been the chemotherapy used to ablate the existing bone marrow as it can be risky and time-consuming.
CRISPR-based therapies are also used in the treatment of blood and lung cancers. In 2016, CRISPR therapy was first used to treat lung cancer; a total of 12 patients participated in the study. The patients were injected with edited T-cells where the PD-1 gene was prevented from making PD-1 receptors. Inhibiting the programmed cell death 1 (PD-1) receptor is beneficial as this receptor mediates the immune escape of tumour cells at the surface of immune cells by activating apoptosis of antigen-specific T-cells and inhibiting apoptosis of regulatory T-cells. At the end of the study, the researchers concluded that the side effects were acceptable and low levels of edited T-cells were still present in 11 out of the 12 patients at two months after the infusion.
The next study into CRISPR-based therapy ended in 2020 and focused on the safety of the treatment and the potential side effects in two volunteers, who suffered from white blood cell cancer and metastatic bone cancer respectively. The results were encouraging, with T-cells maintained at stable levels even after nine months and correctly identifying tumours. Clinical trials using CRISPR-based immunotherapies are still ongoing.
CRISPR trials are also being performed to edit a patient’s defective photoreceptor gene to treat Leber congenital amaurosis 10 (LCA10) which is a cause of childhood blindness. In LCA10, a mutation of a photoreceptor leads to the formation of a shortened, defective version of an important protein. With treatment, this gene mutation can be corrected, allowing cells to make full-length, functional proteins again. The first clinical study began in March 2020, when a patient was injected with a low-dose treatment, but no results have been published yet. This is the first in vivo study as no CRISPR-based therapy had used direct injection (in this case into the eye) to edit the patient’s genes before this trial.
Besides all the points mentioned above, CRISPR technology is also used in clinical trials focusing on the treatment of urinary tract infections and hereditary transthyretin amyloidosis (hATTR ).
In the following section, we will describe actual research which exploited the power of the CRISPR-cas9 genome editing in order to treat a disease. The study was carried out on mice. This way the genetic engineering technique had two uses in this experiment. First, the mice needed to be engineered to be suitable models of the disease that occurs in humans. Then this induced mutation was corrected by the same tool. The following text aims to provide a good grasp on the concept and structure of such a study without claiming to be exhaustive.
All the images are from the original publication apart from the case where it is stated otherwise.
A case study on CRISPR-cas9 genome engineering
An extract from the study “In Vivo CRISPR/Cas9-Mediated Genome Editing Mitigates Photoreceptor Degeneration in a Mouse Model of X-Linked Retinitis Pigmentosa” by Shuang Hu; Juan Du; Ningning Chen; Ruixuan Jia; Jinlu Zhang; Xiaozhen Liu; Liping Yang
Retinis pigmentosa is an inherited disorder that causes the progressive loss of photoreceptors and can lead to blindness. X-linked retinitis pigmentosa (XLRP) is due to mutations in Mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene.
This study aimed to treat this disorder through genome engineering with the CRISPR-cas9 system.
Mouse model:
Cre-dependent Cas9 knock-in mice were generated by inserting a Cas9 transgene expression cassette into a locus. This transgene was controlled by the combination of a cassette (loxP-stop-loxP) that interrupted the transgene and the Cre recombinase, which duo is commonly used to control gene expression. In this case Cas 9 cannot be expressed without the Cre recombinase.
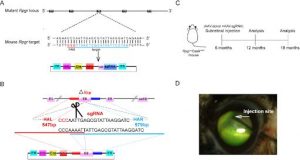
These Cre-dependent Cas9 knock-in homozygous males were crossed with genetically engineered knockout (Rpgr KO) female mice that had a five basepair deletion in exon eight. This crossing provided the mice to experiment on, the Rpgr−/yCas9+/WT male mice.
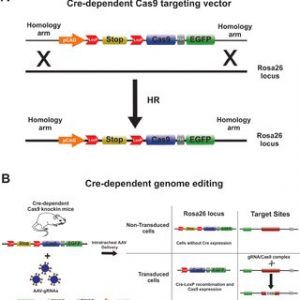
Figure adapted from: Rocha-Martins, Maurício & Cavalheiro, Gabriel & Rodrigues, Gabriel & Martins, Rodrigo. (2015). From Gene Targeting to Genome Editing: Transgenic animals applications and beyond. Anais da Academia Brasileira de Ciencias. 87. 1323-1348. 10.1590/0001-3765201520140710.
The wild type (WT) mice used in the study were C57BL/6J (a commonly used species in research)
Generation of Rpgr KO Mouse Model:
Two sgRNAs targeting exon eight of the Rpgr gene.
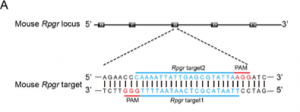
The mRNA of in vitro transcribed Cas9 and sgRNA were injected into zygotes of C57BL/6J mice.
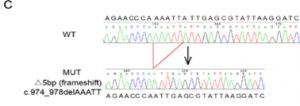
Sanger sequencing analyses showed which offspring managed to keep the desired mutation:

Mice with a 5-bp deletion in exon eight were selected to carry on the project with.
Six or 12 months after treatment, the animals were euthanized, and the eyes were harvested.
Immunofluorescence, morphometric studies, and fundus photography were carried out and the results were analyzed.
Immunostaining of the gene in question was carried out, and the result can be seen on the following images. It shows no staining in the engineered mice, which indicates that the Rpgr gene had been inactivated by the five bp deletion.
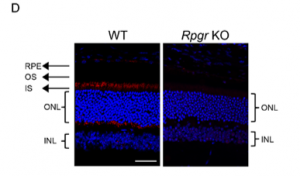
Photoreceptor Degeneration in Rpgr KO Mice
Delayed and slow retinal degeneration was observed in genetically the Rpgr KO mice and histological studies proved the loss of photoreceptor cells in them as well.
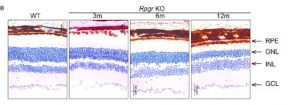
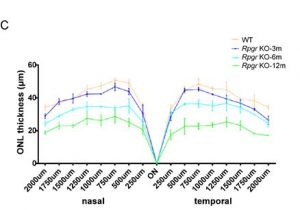
The graphs that were plotted based on the ONL thickness at different points along the optic nerve show significant differences between the control and the engineered mice.
Abnormal Photoreceptor Protein Expression in Rpgr KO Mice:
Mouse cone photoreceptors express cone opsins which therefore can be used as markers. Peanut agglutinin (PNA) is a molecule that binds to cone cells, therefore, can be also used as a marker. Staining for these showed the progressive increase of these proteins and retinal degeneration.
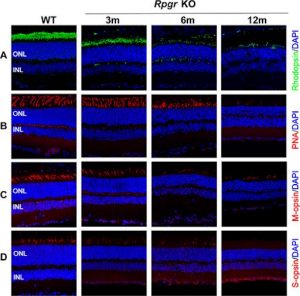
In conclusion we can state that this Rpgr KO mouse model provides an appropriate animal model system for gene editing therapy study.
Photoreceptor Preservation Following Cas9-Mediated Gene Editing Therapy
The aim was to correct the five bp deletion through cutting the deficient region and insert the missing bases through homologous DNA repair. The genetic engineering machinery is transferred into non-dividing photoreceptor cells of organisms by Adeno- Associated Virus (AAV) vectors, more precisely AAV vectors, which target specifically photoreceptors. (AAV2/8 vector) The sgRNA targeted the 5-bp deletion in Rpgr KO mice. The 5′ and 3′ homology arms were amplified from the C57BL/6J mouse genome. The RPGR-5′HA and RPGR-3′HA overlap was subcloned into a plasmid. The Cre recombinase was added as well to activate the Cas9 gene.
Expression cassettes of sgRNA targeted to the mutant Rpgr locus and donor template were delivered to 6-month-old Rpgr−/yCas9+/WT mice by the AAV2/8 vector vectors.
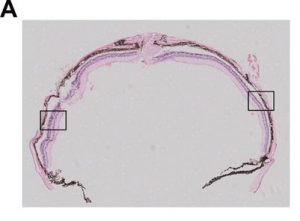
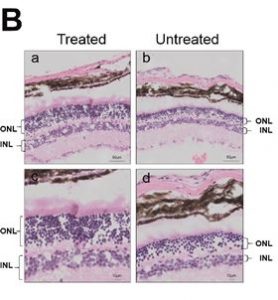
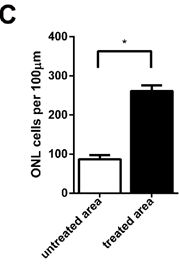
The gene therapy treated mice’s retina was analyzed and assessed six months later: As can be seen on the images below up to nine layers of photoreceptors had been preserved in the treated parts of the retina, while they could be found across four degenerated layers in the untreated parts of the retina of the same eye.
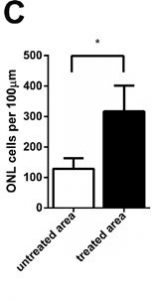
There were 1.5-fold more outer nuclear cells in the treated part of the retina than in the untreated part.
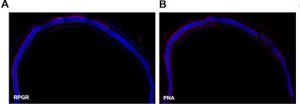
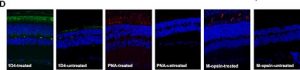
Immunostaining was carried out again and indicated significant Rpgr expression in in the treated areas and no expression in the untreated areas as it can be seen in the images below.
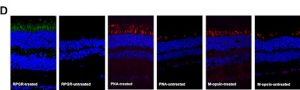
Retinal morphology of Rpgr KO mice was assessed 12 months after treatment as well in order to investigate the long-term impact of gene therapy. Again significant, Rpgr staining and PNA staining were observed.
The density of photoreceptors in the treated area was three-fold greater than that of the untreated area.
Both PNA and M-cone expression in the treated area of the Rpgr KO mice were similar to that of 6-month-old untreated Rpgr KO mice, with rhodopsin expression similar to that of 3-month-old untreated Rpgr KO mice.
These data suggested that CRISPR/Cas9-mediated Rpgr gene editing therapy successfully preserved photoreceptors, and this effect seemed to be persistent.
Sources:
Hu S, Du J, Chen N, et al. In vivo CRISPR/Cas9-mediated genome editing mitigates photoreceptor degeneration in a mouse model of X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2020;61(4):31. https://doi.org/10.1167/iovs.61.4.31
Henderson, H., 2021. CRISPR Clinical Trials: A 2021 Update. [online] Innovative Genomics Institute (IGI). Available at: <https://innovativegenomics.org/news/crispr-clinical-trials-2021/> [Accessed 30 August 2021].
Nast, C., 2021. This is the year that CRISPR moves from lab to clinic. [online] WIRED UK. Available at: <https://www.wired.co.uk/article/jennifer-doudna-crispr> [Accessed 30 August 2021].
Han, Y., Liu, D., & Li, L. (2020). PD-1/PD-L1 pathway: current researches in cancer. American journal of cancer research, 10(3), 727–742. Available at: <https://www.ncbi.nlm.nih.gov/ pmc/articles/ PMC7136921 > [Accessed 16 September 2021].